News Release
Arrhythmia mapping technology demonstrates positive clinical results
May 4, 2022--Bioengineers and cardiologists from the University of California San Diego invented a technology that can accurately and noninvasively map atrial and ventricular heart arrhythmias in a matter of minutes. The technology, developed by Vektor Medical Inc., a company co-founded by Â鶹´«Ã½ faculty, demonstrated 97.3 percent accuracy in a clinical validation study, and recently received FDA clearance.
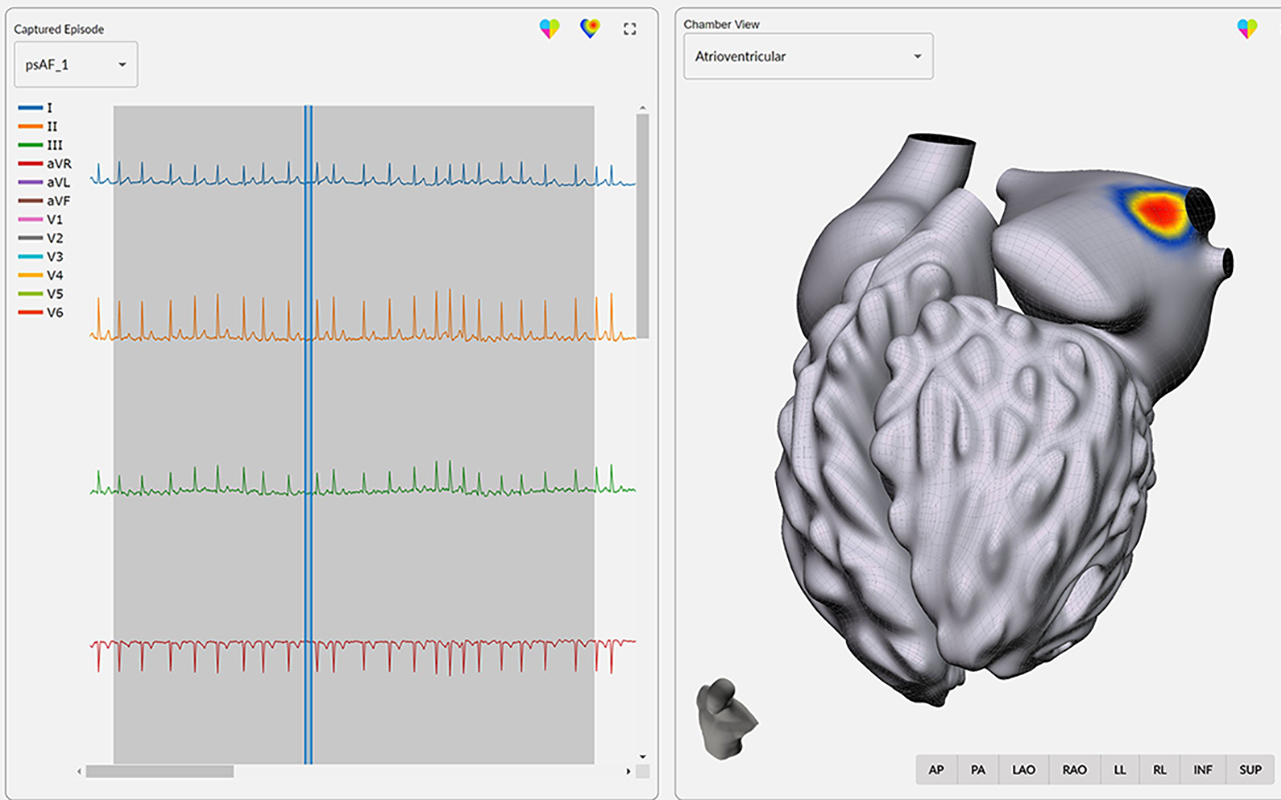 |
| The vMap technology localizes the source of arrhythmia using only data from a noninvasive 12-lead ECG. |
Instead of sending a catheter into a patient’s heart to localize the source of the arrhythmia, the new vMap technology requires only data from a standard noninvasive 12-lead electrocardiogram (ECG) – performed in most clinical and ambulatory settings – to create a three-dimensional interactive map of arrhythmia source locations in all four chambers of the heart.
The researchers used computational modeling to create over one million simulations of different heart arrhythmias. The patient’s ECG recordings are compared to this simulation database to accurately locate the source of the arrhythmia.
vMap was invented by bioengineers and cardiologists from Â鶹´«Ã½, and has been developed by Vektor Medical, a startup co-founded by Professor of Bioengineering, Andrew McCulloch; Professor of Medicine and electrophysiologist, Dr. David Krummen; Assistant Professor of Medicine, electrophysiologist, and bioengineering alumnus, Dr. Gordon Ho; and bioengineering alumnus Dr. Christopher Villongco, Vektor’s chief technology officer. The Vektor Medical team presented their clinical trial results at the Heart Rhythm Society meeting in San Francisco.
Pinpointing an electrical fault
 |
| David Krummen, MD, cardiac electrophysiologist at Â鶹´«Ã½ Health and professor of medicine at Â鶹´«Ã½ School of Medicine, is a co-inventor of vMap. |
The current standard of care for treating heart arrhythmias – any type of irregular heartbeat that is too fast, too slow, or mistimed due to an electrical signal misfiring – is to carefully burn or freeze the exact spot in the heart where the electrical misfiring is coming from. This procedure, called an ablation, resets the electrical signals and heartbeat. Hundreds of thousands of ablations are performed in the United States every year. But before an electrophysiologist can perform an ablation, they need to know precisely where in the heart the arrhythmia is coming from.
“Mapping is critical to planning and performing ablation. The current process of arrhythmia localization relies upon invasive catheter mapping” said Dr. David Krummen. “It generally works well, but the process can be time consuming, increasing fluoroscopy and anesthesia exposure for the patient. Moreover, occasionally the catheter-based process may "bump" the arrhythmia source and suppress the clinical arrhythmia, preventing further mapping.”
“We wanted to find a way to pinpoint the arrhythmia source location using data from the 12-lead ECG,” he added. “The 12-lead ECG is used in a variety of clinical settings outside the electrophysiology laboratory including the emergency department, the intensive care unit, and the outpatient clinic. One of the major goals of the system is to leverage arrhythmia data from these ECGs to potentially allow arrhythmia source mapping prior to arrival in electrophysiology lab.”
The computational solution
That’s where bioengineering Professor Andrew McCulloch and Christopher Villongco, a PhD student in McCulloch’s lab at the time and now Vektor’s CTO, came in. McCulloch has spent the last 35 years at Â鶹´«Ã½ making models of the heart, from the biochemistry and physiology of its cells and the mechanics of its muscle fibers to the electrical rhythms of the whole heart. His cardiac computational modeling software, Continuity, allows researchers to accurately simulate the heart down to the cellular level. Villongco and McCulloch originally set out to develop personalized computational models to help patients and physicians predict the outcome of certain heart procedures, but soon hit a stumbling block: how to translate computational modeling from academic use to real-time, actionable clinical use?
“Developing detailed, personalized models for individual patients was appropriate for our research at the time, but the process of creating a single model requires a lot of high-quality clinical data, computational power, time, and modeling expertise,” said Villongco. “We learned from collaborating with Drs. Krummen and Ho that the actual clinical demands were quite the opposite: minimal data, limited computational power, rapid analysis, and ease of use in the existing clinical workflow.”
 |
| Andrew McCulloch, a professor of bioengineering and Shu Chien Chancellor’s Endowed Chair in Engineering and Medicine, has spent the last 35 years at Â鶹´«Ã½ modeling the heart. |
Learning from the collaboration, the team realized they needed to create a massive library of computational models of arrhythmias.
Then the challenge became how to create a model library, when it took a powerful computer cluster several days to create just one model? Combining computationally efficient modeling methods with modern-day computing architectures and resources, a library of over one million arrhythmia simulations was created in just under one year, a vast improvement from the tens of thousands of years it would have otherwise taken. The clinical product was then designed and implemented according to the needs and experiences of electrophysiologists.
The initial research was funded by grants from the NIH, a Jacobs School of Engineering entrepreneurship program, and the Galvanizing Engineering in Medicine program. Then, thanks to funding from the NIH-backed Center for Accelerated Innovation – intended to help transfer technologies from the lab to the clinic – the researchers and Vektor team were able to further hone the tool, expand its capabilities, and test its accuracy. A multicenter, blinded clinical trial showed that in 255 arrhythmia episodes from 225 patients, the vMap tool had a regional accuracy of 96.9%, with a spatial accuracy of 15mm. The median time to complete the mapping process was 48 seconds.
“It’s one thing to make fancy simulations, but it’s altogether another to make something that can be used in the pressure of a clinic, in a matter of minutes, that’s reliable,” said McCulloch. “And we’ve shown that vMap meets all these needs.” McCulloch is also the director of the Â鶹´«Ã½ Institute for Engineering in Medicine, and the Shu Chien Chancellor’s Endowed Chair in Engineering and Medicine.
vMap is a tool for physicians to better understand seven important and often dangerous arrhythmias – ventricular tachycardia; premature ventricular complex; ventricular fibrillation; focal atrial tachycardia; premature atrial complex; orthodromic atrioventricular reentrant tachycardia; and atrial fibrillation. The information vMap provides is intended to increase the success rate for patients undergoing these ablation procedures.
vMap is currently in use by physicians at Â鶹´«Ã½ Health with the intent to reduce ablation procedure times and increase ablation success rates for patients with these seven arrhythmias.
Disclosure: Andrew McCulloch, Ph.D. is a professor of bioengineering at Â鶹´«Ã½ Jacobs School of Engineering. Dr. McCulloch is an inventor of vMap and has equity as a co-founder and Scientific Advisory Board member to Vektor Medical, Inc.
David Krummen, MD, is a cardiac electrophysiologist at Â鶹´«Ã½ Health and professor of medicine at Â鶹´«Ã½ School of Medicine. Dr. Krummen is inventor of vMap and has equity as a co-founder and clinical advisor to Vektor Medical, Inc.
Media Contacts
Katherine Connor
Jacobs School of Engineering
858-534-8374
khconnor@ucsd.edu